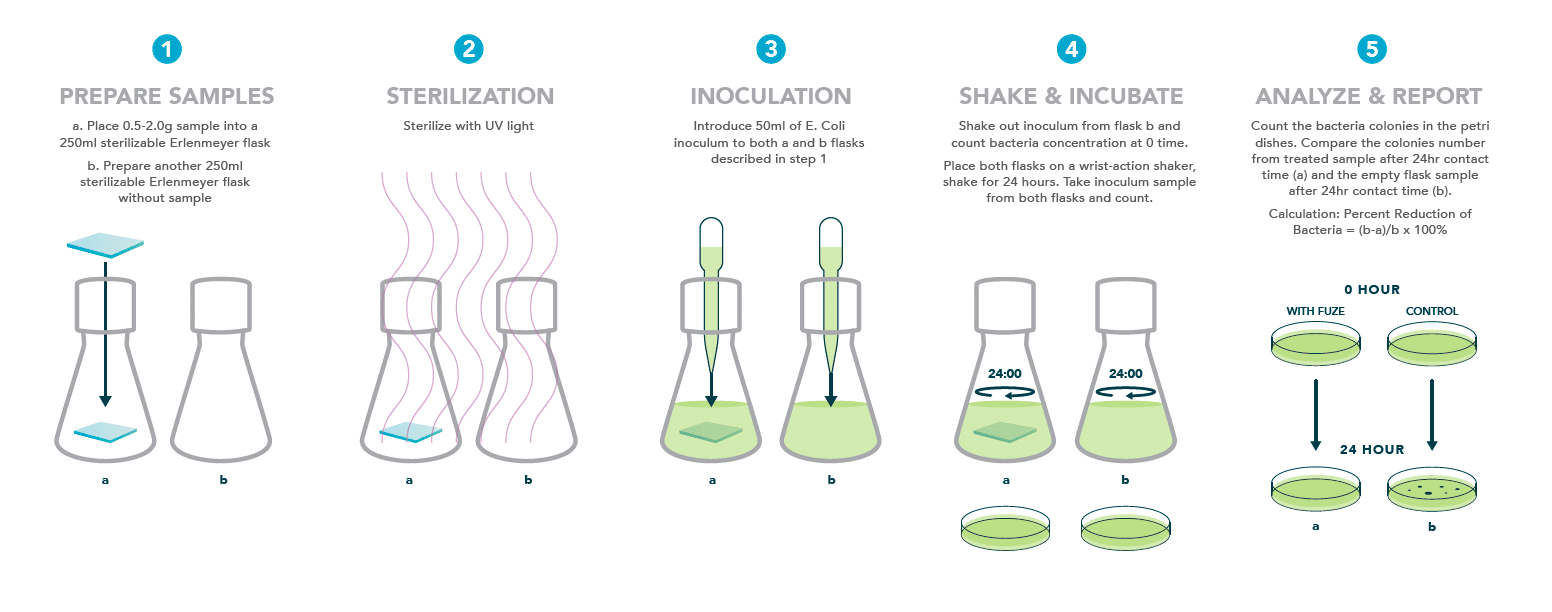
Textile efficacy testing for non-leaching antimicrobial agents: ASTM E2149-13a
Procedure: The sample is sometimes sterilized by labs with either UV or autoclave. The test microorganism is grown in a liquid culture. The microbial culture is then diluted in a sterile buffer solution. For each product to be tested, 50 mL of the standardized microbial culture is placed into three sterile glass containers. One container receives only the bacterial suspension, another receives the antimicrobial test object, and the last receives a control object. Microbial concentrations are enumerated at “time zero.” All containers are shaken in a wrist action shaker for a specified contact time, usually 1 to 24 hours. After the specified contact time, the microbial concentration in all containers is determined and compared to the “time zero” concentration as well as the control.
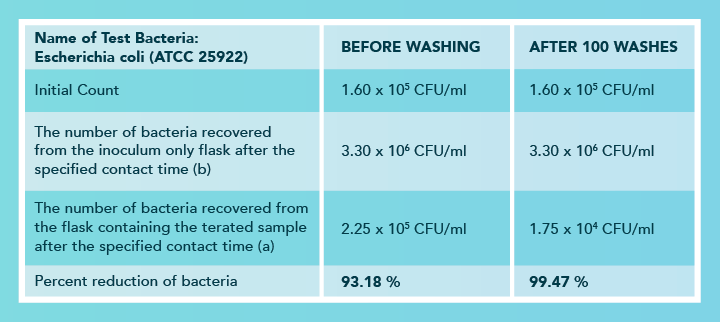
Unlike most other antimicrobial treatments that wash away and decrease efficacy when washed, FUZE technology improves with washing when dirt and softeners are removed and allow for bacteria to come in contact with FUZE faster!

FUZE obliterates harmful and odor-causing pathogens by mechanically disassembling organisms upon contact and rendering it incapable of multiplying!
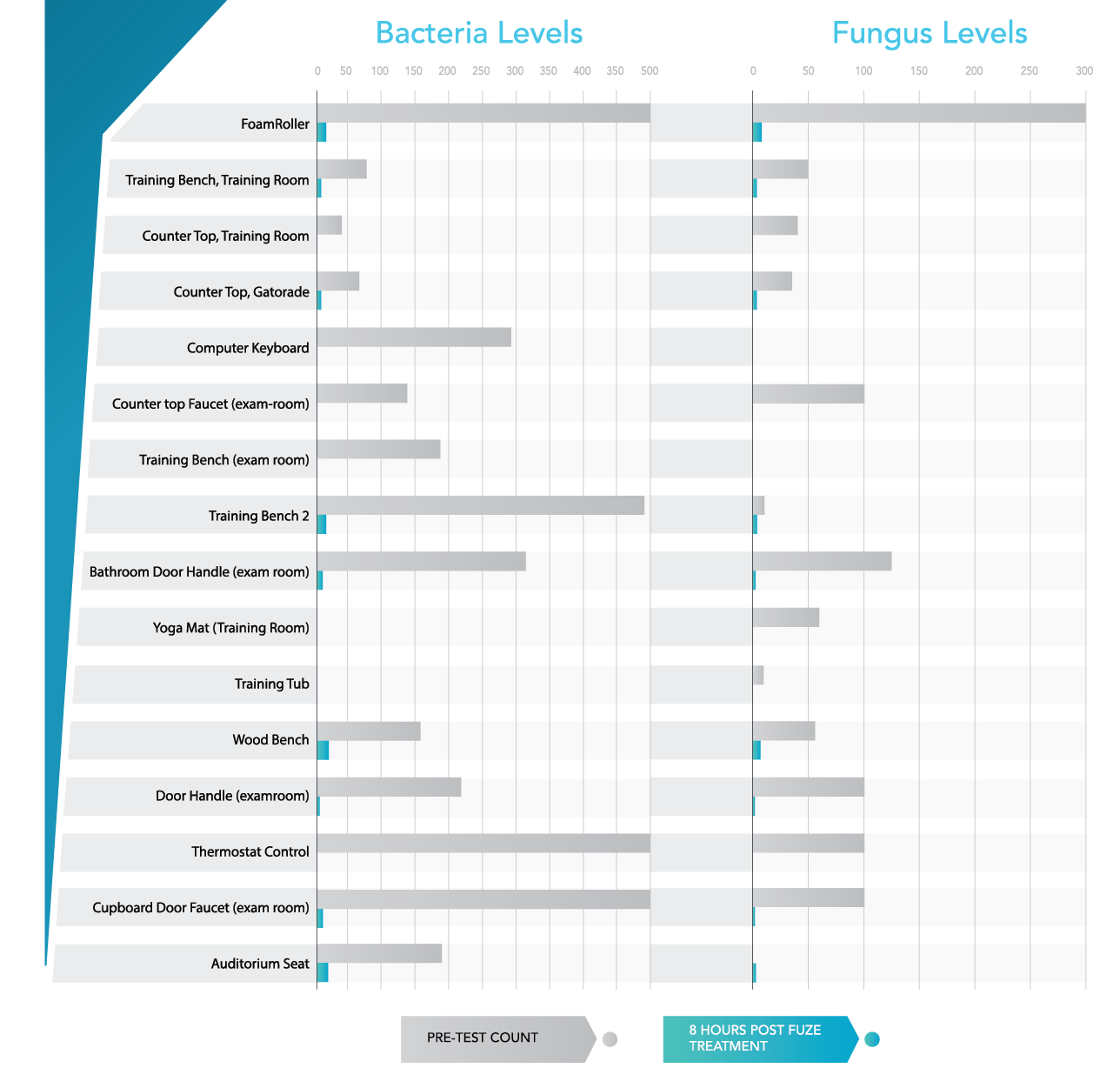
We achieved our goal of protecting the New England Patriots athletes and employees by eliminating harmful pathogens at targeted areas of Gillette Stadium via our chemical free application. Bacteria and fungus levels were reduced by >99% after the Fuze treatment. CFU counts in all the target areas of Gillette were either completely eliminated or found to be below the certified danger level of 50 CFU’s.


If you’d like more information, would like to request a sample, or would like a quote. Please fill out our contact form with your request and we will get back to you as soon as possible.
